|
||||||||||||||||||||||
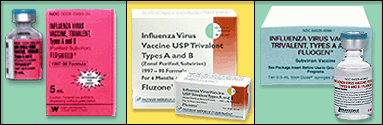
The vaccines used in Canada now are called split-virus preparations. They are treated with an organic solvent to remove surface glycoproteins and thus reduce vaccine reactogenicity (and the rate of side effects). Split-virus and whole-virus vaccines are similar with respect to immunogenicity, but split-virus vaccine is generally associated with fewer side effects. Vaccines are not approved for infants less than 6 months of age. The effectiveness of influenza vaccine varies depending upon the age and immunocompetence of the vaccine recipient and the degree of similarity between the virus strain included in the vaccine and the strain of virus causing the infection.Over the last decade, improved global surveillance activities for influenza have resulted in the use of vaccine strains that are closely matched with circulating strains. With a good match, influenza vaccination has been shown to prevent illness in 70-80% of healthy children and adults, and 50% of elderly, community living adults. Studies among elderly nursing home residents have shown influenza vaccination to be 50% to 60% effective in preventing hospitalization and pneumonia, and 85% effective in preventing death, even though the efficacy in preventing influenza-like illness is only 30% to 40%. Vaccination for preventing influenza is generally recommended for the following high risk groups:
Vaccination can be given to other people like working adults, pregnant women, travelers to areas where the influenza virus is likely to be circulating or more liberally to anyone in the population greater than 6 months of age. The most common side effect from influenza vaccination is soreness at the site of the injection. Less common side effects that can occur after vaccination include allergic reactions. Guillain-Barre syndrome (GBS), a rare neurologic illness has been estimated to be about one additional case of GBS per 1 million doses of vaccine given. Influenza vaccine is contraindicated for anyone with a serious allergy to eggs or egg product. A live attenuated, cold-adapted, nasal spray vaccine has
undergone successful trials and has demonstrated efficacy
that is as good or better than traditional inactivated vaccines.
The intranasal delivery system is particularly attractive
for the pediatric populations.
Large-scale prevention methods
|
||||||||||||||||||||||
Surveillance / Infection Control
Currently, a monitoring network of sentinel doctors has been established in Canada. When they suspect a patient may have influenza, they send a nasopharyngeal swab from that patient to a central laboratory for analysis. These laboratories confirm influenza and determine its type and subtype.
The flu season is declared started when more than two cases are confirmed in one week in any one practice. The data are reported to national public health laboratories, who then alert national health organizations and the media, who in turn inform the public and keep them informed as to the flu's progress across Canada. These data are also reported to the World Health Organization (WHO).
The Laboratory Centre for Disease Control (LCDC) at Health Canada collaborates with this network of laboratories to identify and characterize strains of influenza virus. This network can determine what are the prominent strains and use this information to predict future "up and coming " strains for inclusion into future vaccines.
The viruses identified are reported to the four WHO Collaborating Centres for Virus Reference and Research in Melbourne, Tokyo, London, and Atlanta. In this network are included 110 National Influenza Centres in 83 countries. This network helps WHO monitor influenza activity in all regions of the world and ensures that virus isolates and information are sent rapidly to the WHO Collaborating Centres for Virus Reference and Research for immediate strain identification.
For many years, WHO, has conducted consultations to review the currently circulating influenza virus strain worldwide and to recommend the appropriate vaccine composition to be used for next season. WHO has also developed a plan for the global Management and Control of an Influenza Pandemic. Elements of this plan include augmented surveillance for and identification of potential pandemic viruses, dissemination of information, logistic and other support to national health authorities, and the promotion of high growth seed virus for vaccine and facilitation of vaccine production and international distribution. This international surveillance system will provide Canada in a case of pandemic, with an early warning, in order to start vaccine production as quickly as possible. Immunization will be the only way to minimize the impact of the pandemic on Canadians.
Health Canada is working with provincial and territorial
governments to finalize a contingency plan which would provide
the basis for coordinated and collaborative action by the
provinces as well as Health Canada and other agencies in the
event of a pandemic.